Did you know that CTEPH could develop after PE?
CTEPH is an infrequent, but feared, potential long-term complication of acute PE1
Between 2.8–3.2% of patients may go on to develop CTEPH after surviving an acute pulmonary embolism (PE).2 However, 25–30% of patients with CTEPH have no history suggestive of acute PE; therefore CTEPH should still be considered in patients without previous acute PE.3,4
Approximately 3% of survivors of acute PE develop CTEPH.2
Identification of PE patients at risk of developing CTEPH
Although routine screening for CTEPH is not currently recommended in asymptomatic survivors of PE, patients should be screened to exclude CTEPH if they have:5,6
-
A history of PE and
-
Persistent/exercise-induced dyspnea after a minimum of 3 months of effective anticoagulation
Various factors are predictive of CTEPH in patients following an acute PE:1
-
Recurrent or unprovoked (idiopathic) PE
-
Known hypothyroidism
-
Symptom onset >2 weeks before PE diagnosis
-
Right ventricular dysfunction on CT or echocardiography
If CTEPH is suspected, patients should be referred to a CTEPH expert center to confirm the diagnosis and assess their suitability for pulmonary endarterectomy (PEA) surgery.6
The pathogenesis of CTEPH is thought to involve incomplete resolution of embolic material7
Although the pathogenesis of CTEPH is yet to be fully elucidated, it is understood to involve incomplete resolution of embolic material as a result of a misguided vascular remodeling process.7
Traditional thromboembolic model of CTEPH progression7–9
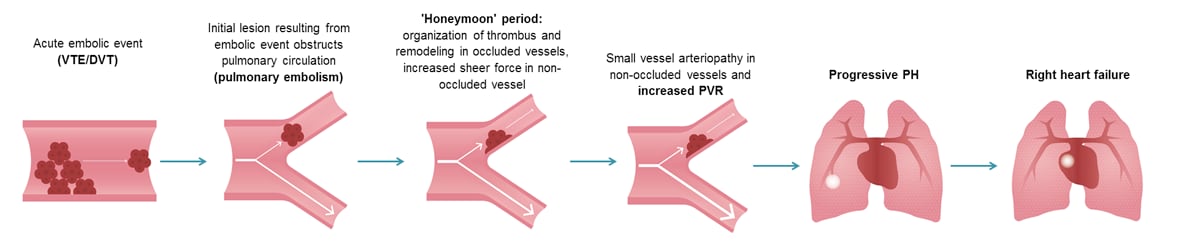
Early diagnosis of CTEPH is warranted since PEA surgery is potentially curative
The first step to CTEPH diagnosis is a high index of clinical suspicion.1
Clinical signs of CTEPH are similar to those seen in other forms of pulmonary hypertension (PH).6 Patients typically present with progressive dyspnea and exercise intolerance occurring several months or even years after the thromboembolic event, and despite effective anticoagulation.5,7,10 Edema and/or signs of right heart dysfunction including fatigue, chest pain and syncope are other common symptoms.11
The nonspecific symptoms of CTEPH, combined with an older patient population and high prevalence of cardiac and pulmonary comorbidities, often contribute to under- or delayed diagnosis of the disease (median delay of 14 months).1,3
Treatment of CTEPH
Around two-thirds of CTEPH patients are eligible for PEA, which is a potentially curative procedure,3 so it is important that all patients are assessed for operability.
Find out more about PEA.
Although PEA represents a surgical cure, around one-third of patients experience persistent or recurrent CTEPH following PEA.12 For these patients, and those who are not suitable candidates for PEA, medical therapy is recommended.6
Riociguat is the only approved treatment indicated for patients with inoperable CTEPH or persistent/recurrent CTEPH after PEA.13 Riociguat has demonstrated long-term benefits in patients with inoperable and persistent or recurrent CTEPH in the large, randomized CHEST clinical trial, as well as in real-world studies.14–16
Find out more about medical therapy.
You may also be interested in

Useful resources
Our resources page contains a range of material to help with the identification, diagnosis and management of CTEPH.
References:
1.Klok FA et al. J Thromb Haemostat 2018;16:1040–51. 2.Ende-Verhaar YM et al. Eur Respir J 2017;49:pii1601792. 3.Pepke-Zaba J et al. Circulation 2011;124:1973–81. 4. Jamieson SW et al. Ann Thorac Surg 2003;76:1457–62. 5. Konstantinides SV et al. Eur Heart J 2014;35:3033–73. 6. Galiè N et al. Eur Respir J 2015;46:903–75. 7. Lang IM et al. Ann Am Thorac Soc 2016;13:S215–21. 8. Peacock A et al. Proc Am Thorac Soc 2006;3:608–14. 9. Simonneau G et al. Eur Respir Rev 2017;26:160121. 10. Hoeper MM et al. Circulation 2006;113:2011–20. 11. Jenkins D et al. Eur Respir J 2012;21:32–9. 12. Edward JA, Mandras S. Curr Probl Cardiol 2017;42:7–38. 13. Bayer. Riociguat Company Core Data Sheet. Version 05. May 2017. 14. Klose H et al. Am J Respir Crit Care Med 2018;197:A5682. 15. Ghofrani HA et al. N Engl J Med 2013;369:319–29. 16. Simonneau G et al. Lancet Respir Med 2016;4:372–80.


